
Vitamin A
On this long page I would like to tell you in detail about vitamin A. But let me start with the prevailing views. Anyone who wonders what vitamin A actually is will quickly find these answers:
Vitamin A is a fat-soluble vitamin and your body needs it for the eyes, skin, hair, nails, immune system, growth and cell division. It is an essential nutrient! Vitamin A deficiency leads to reduced resistance, dry or flaky skin and vision problems. Too much vitamin A is dangerous for pregnant women.
And that's almost all true, except… it's not a vitamin and it's not a nutrient.
The confusion started more than a century ago. A “factor” was discovered in cod liver oil that allowed sick laboratory animals to recover from the growth retardation they suffered as laboratory animals. A new vitamin! But it seemed as if there were two substances that had the same restorative effect on those animals. And eventually that turned out to be the case: there are indeed two substances, one of which is the precursor of the other. But the discovered and named “vitamin” from liver oil turned out to be the ultimate substance that the body produces itself (and not its precursor). It was only established years later that the necessary source (the precursor) was vegetable carotene. Even later it also turned out that there were not two substances, but two groups of substances. In the literature on vitamin A we now come across two terms: carotenoids and retinoids.
Infobox: carotenoids and retinoids
Carotenoids: only occur in the plant kingdom. They give colour to our vegetables and fruit. The name is derived from the Latin word carota, for carrot. And indeed, the orange carrot contains the best-known carotenoid beta-carotene, which is also the most common precursor of vitamin A. The word precursor indicates that this substance is needed to be able to produce the next substance from it. Without carotene, animals cannot produce vitamin A.
Retinoids: this is the name of the substances that resemble retinol, the substance that was designated as vitamin A. Retinoids occur in nature only in the animal kingdom. They are so-called endogenous substances, they are created within the organism. To produce retinoids, carotene is needed as the source, from something plant-based that is eaten. Retinoids can also be synthetic, they are then made in a chemical factory. These agents are used, for example, as medicine or in skin care products. Or they are added to foodstuffs, or sold as a supplement.
Now you may be wondering: if carotene is a substance “essential for the maintenance of normal metabolic functions, but is not synthesized in the body and must therefore be obtained from an exogenous source”, then thát is the vitamin, right? 1) Shouldn’t then these carotenes be designated as such? Yes! But that is not how it has historically worked out.
Two Mistakes
In the late 19th and early 20th centuries, several scientists (Lunin, Socin, Pekelharing, Hopkins, etc.) conducted experiments in which their test animals were not given vegetables or fruit. The animals (cows, goats and rodents, all herbivores) suffered from stunted growth, even though they were given sufficient food according to the prevailing insights. It soon became apparent that a very small amount of milk brought recovery.
Edwin Hart, Elmer McCollum and Harry Steenbock reported in 1917 on yellow corn, a vegetable source that also seemed to contain this restorative factor. Cows (which mainly eat grass) appeared to become ill if they were only given wheat. Wheat contains very little carotene. However, if part of the feed was given as yellow corn, the adverse effects of wheat appeared to be eliminated. Alfalfa (lucerne) hay also appeared to work very well for the grass eaters. 2) In 1919, Steenbock wrote that he had also found the factor in sweet potato and (orange) carrots. 3)
Infobox: Thomas Moore looks back
All the test animals used were herbivores and various plant sources could provide the restorative factor. These foods usually had a yellowish/orange colour. Nevertheless, a substance from cod liver oil, of animal origin, was designated as the vitamin. A stable form of this “vitamin” could be obtained from cod liver oil, while this was very difficult to obtain from plants.
In 1929, Thomas Moore demonstrated that this animal factor (retinol) is formed by the animal from the plant carotene. He announced his findings in the prestigious medical science journal The Lancet. 4) In 1930 he published an extensive article about the discovery. 5)
By the time Moore provided his proof, the first vitamin preparations had long been marketed by pharmaceutical companies. 6) Parke, Davis and Company, a pharmaceutical firm that’s now a subsidiary of Pfizer, had already in 1920 launched Metagen, a 325mg capsule containing the known fat-soluble vitamin A. 7) The wrong assumption had caused too much delay, his discovery came too late. Moore also mentions Takahashi's report on the toxicity of overdosing on vitamin A in his article. In a scientific journal from 1941 he looks back on that time. 8)

As Thomas Moore relates in his article, it was mistakenly assumed that carotene “was devoid of biological activity”. An error that contributed to retinol becoming the designated vitamin and not carotene (or actually carotenes, because there is also alpha-carotene and beta-cryptoxanthin). However, it is clear that vitamin A is not a vitamin at all but a substance produced by the body. Thomas Moore showed that beta-carotene is converted to vitamin A (retinol) in the body. 9)10) Nevertheless, the easily isolable and stable retinol was held up as “the real vitamin A”.
But then another wrong assumption was made. It was assumed that the ingested beta-carotene is always completely converted into vitamin A, which would then be stored in the liver as a reserve. However, that is not the case, the conversion of beta-carotene is automatically limited. It is even the case that your skin turns orange from the pigment carotene when you ingest a lot of it. Precisely because not everything is converted into the virtually colorless retinol, but is stored in subcutaneous fat, you get a tan from it (carotinemia). After so many carrots, the conversion stops itself. Was such a feedback control system too difficult a concept at the time? After the Second World War?
In 1949, a specially appointed committee was given the task of determining the correct conversion factor for the conversion of beta-carotene to vitamin A. This proved to be a very difficult, virtually impossible task. However, the report that was drawn up on this subject shows no doubt about the principles and feasibility of the task. The fixed conversion factor was eventually officially recorded as if it could exist. A second mistake. There was a lot of confusion.
Infobox: The Vitamin A Subcommittee's Confusion
For the international conference that the newly formed World Health Organization was to hold in 1949, a “Vitamin A Subcommittee of the Medical Research Council's Accessory Food Factors Committee” was appointed. The intention at the conference was to change the standard for vitamin A from natural beta-carotene to a synthetic retinyl ester. The committee was charged with determining the conversion factor from beta-carotene to vitamin A.
If we look at the structure of those two molecules, the answer seems very simple: just cut that beta-carotene in half and you have twice as many molecules of vitamin A. In 1941, Thomas Moore drew those two molecules by hand.
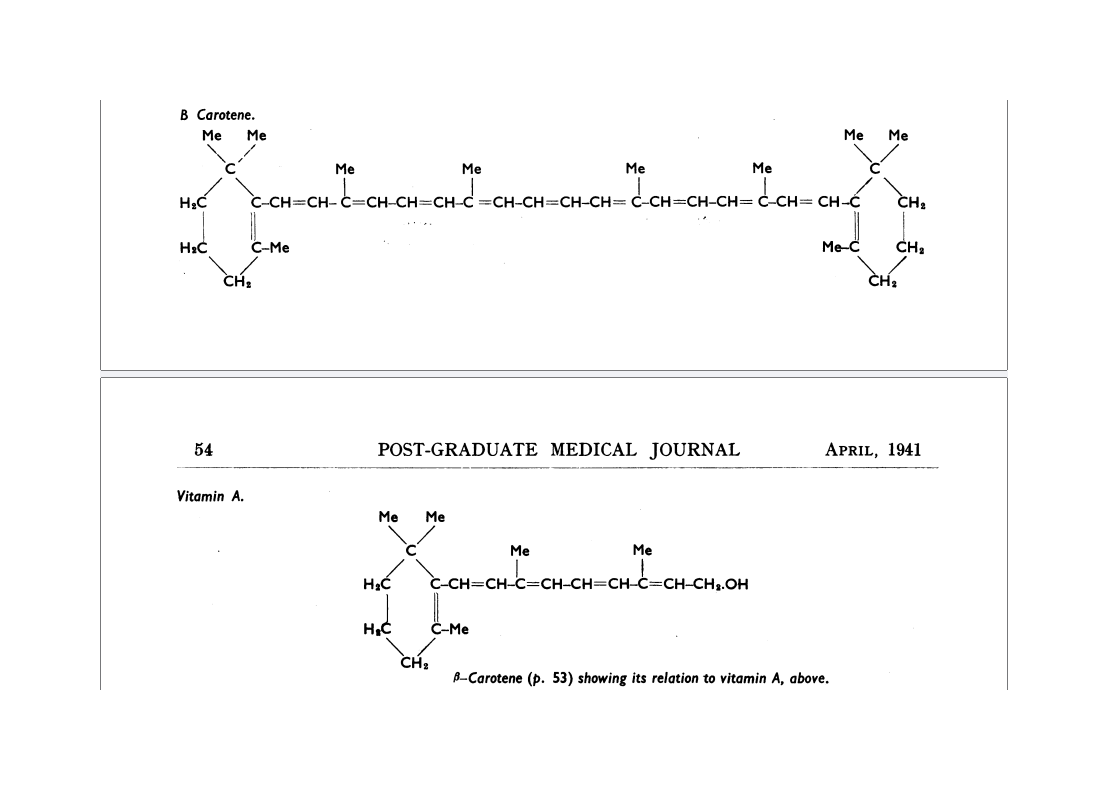
The committee expected that beta-carotene would be more active than vitamin A, because this splitting increases the number of molecules. But to the surprise of the vitamin A subcommittee, the average result of all the studies showed the opposite. In the report that the secretary of the committee wrote in 1951, the following can be read.
Out of this long research on the magnitude of the conversion factor comes clearly a conclusion of fundamental importance. If 0.3 microgram of vitamin A has the same activity as 0.6 microgram of β-carotene, which is the essential biological result, then the molecule of β-carotene does not split into two molecules of vitamin A as was long supposed, but produces only one. At any rate that is the average result of all the four collaborative researches which have been mentioned. There is some evidence that conditions exist in which it is not always so, but in the conditions of the collaborative experiments it was so.
There was also surprise at the results of all the studies that were conducted because they were completely different from what was expected. But there was no going back to the drawing board, despite “a conclusion of fundamental importance” and the existence of conflicting evidence. The food industry stood ready to add synthetic retinyl esters to margarines.
Unique Vitamin
Vitamin A is unique! We must take just enough and certainly not too much of this very special vitamin in order not to poison ourselves! Or rather:
Vitamin A is unique among the vitamins in that its concentration must be within a very narrow range in order to avoid both deficiency and toxicity. 11)
This can be read in a 2008 study on vitamin A and cell differentiation in the journal Cell. However, the right way to obtain vitamin A is obviously to have our bodies make it themselves from carotene. If we leave it to our body and feed it in the right way, then it will just work out fine. Vegetables and fruit provide beta-carotene, the precursor that was so difficult to isolate and keep stable.
Thomas Moore described it in his retrospective in 1941. He also wrote the following in that article. “In most vegetable tissues the pigment is remarkably stable, and withstands cooking, canning, or the drying of the vegetable under careful conditions.” If we just leave it in the carrots and the apples, it remains very stable. Even canned tomatoes or pizza sauce still provide that “pro-vitamin” and our bodies do the rest. Just right. Without poisoning us.
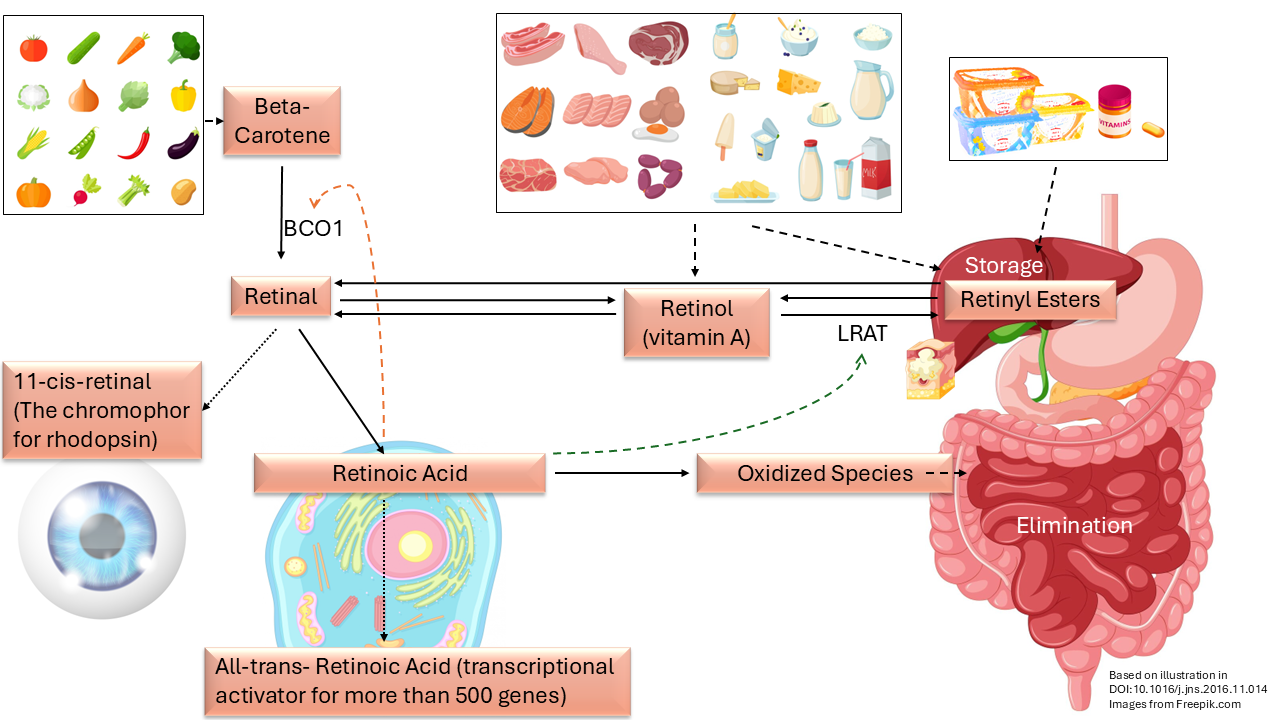
In the meantime, vitamin A metabolism has been increasingly studied. The image above is based on an illustration in a scientific article from 2017. 12)13) It shows the metabolism around vitamin A. Top left we see plant sources of beta-carotene that we ingest with our diet. With the help of an enzyme (BCO1plugin-autotooltip__defaultBeta-carotene 15,15'-dioxygenase) beta-carotene is converted into retinal which is central in the picture. 14)
Infobox: vitamin A metabolism explained further
Retinal can be further converted and used in three ways:
Firstly, it can serve as the light-sensitive substance for our vision in the 11-cis form of retinal. If there is a deficiency of this chromophore, rhodopsin can no longer function and we see worse when it gets dark. Night blindness is one of the first symptoms of “vitamin A deficiency”.
Secondly, in the cells retinal converts to the retinoic acids for gene expression. It is important that the information in our DNA is used correctly for the production of proteins; the building blocks, but also for all kinds of enzymes and antibodies that our body needs in order to function.
15) Thirdly, it can be converted into a retinyl ester via retinol and with the help of an enzyme (LRAT) and then safely stored in the liver or in subcutaneous fat and slowly removed. The storage could also serve as a buffer.
The picture shows how some of the retinoids can be converted into each other bi-directionally, i.e. in two directions, by the organism. The liver is used for long-term storage and can retain a remarkable amount. Moore writes about this in his retrospective that a rat can store for a centuryplugin-autotooltip__default plugin-autotooltip_big"Thus in the rat the amount accumulated may be enough theoretically to suffice normal requirements for a century, although the life span is only three years.". Those rats most likely got the stored retinol directly from cod liver oil and in much larger quantities than was good for them. The logical reason to store so much is of course to prevent poisoning for as long as possible.
The liver can form retinol from the stored retinyl esters, which can then become retinal in the cells. This bidirectional conversion and storage can explain how the researchers were able to let their suffering, malnourished, hypovitaminotic and herbivorous test animals recover with liver oil. So with the retinoids that another animal had produced from carotene and stored in that liver!
However, those malnourished animals were all herbivores, plant eaters. If we look at the picture carefully, we see that retinol (vitamin A) is not necessary from food at all. It is part of the metabolism, but it is not an “essential nutrient”!
For non-herbivores, retinoids come with animal food products from the animal that served as the food source. These extra retinoids are added to the stored retinyl esters in the liver. The unwanted surplus is then removed with the bile (green in the picture). However, the removal of the retinoids takes much longer than the safe storage. As a result, it piles up; it “bio-accumulates”.
In nature, herbivores produce retinoids for their own metabolism from beta-carotene. This conversion is regulated and limited. However, animals in the barn are also directly offered retinoids (vitamin A in the form of synthetic retinyl esters in feed or water). Via animal fats (such as milk fat) and eggs, but also via liver products, they ultimately end up in our diet. In addition, we also ingest the same artificial esters, usually retinyl acetate or retinyl palmitate, directly through additives to products and through any supplements or vitamin preparations. Retinyl acetate is produced completely synthetically on an industrial scale with annual yields of more than 7,500 tons. 16)
Infobox: the synthetic production of vitamin A
It was the two Dutchmen Josef Arens and David van Dorp who first synthesized vitamin A at Organon in Oss, The Netherlands in 1946. But it was Otto Isler at Hoffmann-La Roche in Switzerland who made important advances, so that production could be done on an industrial scale.
In 1948, production of the ester vitamin A acetate was started. The vitamin A factory still forms the heart of DSM-Firmenich in Sisseln, Switzerland; in 2003, the Swiss Roche Vitamins was bought by the Dutch DSM.

The vitamin A that DSM produces is now made from acetone and acetylene. In the end result with a very high yield, the atoms are neatly pushed together to form vitamin A.
This chemical feat is described in detail in 75 Years of Vitamin A Production. The images in this box are taken from that publication under CC-BY-NC-ND 4.0.
Our bodies are forced to store more and more in our livers. This storage averts the consequences of poisoning for as long as possible. It seems that when we are born, we start with a reasonably intact, clean liver. In infants, values were measured from zero, but in the children from the following study it appeared that the numbers were already increasing:
The mean liver stores of vitamin A in children (1 to 10 years of age) have been reported to range from 171 to 723 µg/g (Flores and de Araujo, 1984; Mitchell et al., 1973; Money, 1978; Raica et al., 1972; Underwood et al., 1970), whereas the mean liver vitamin A stores in apparently healthy infants is lower, ranging from 0 to 320 µg/g of liver (Flores and de Araujo, 1984; Huque, 1982; Olson et al., 1979; Raica et al., 1972; Schindler et al., 1988). 17) (p95)
Regulated System
Later studies confirm the proposition that carotenoids (such as beta-carotene) are converted in a very controlled manner. Just as a room thermostat regulates the temperature in our home, there is also a feedback loop in our body that ensures that no more carotene is converted to retinol than the body can use. As more is converted, that conversion is also reduced more. In the picture above, that feedback loop is depicted with the dashed red arrow. By using this control loop, our body ensures that we cannot poison ourselves by eating carrots and kale.
Infobox: The tightly regulated vitamin A metabolism
The quotes below are from a scientific paper explaining how “β-carotene absorption and bioconversion are tightly controlled by body needs”. 18)
Several studies indicate that the vitamin A status of the host affects carotenoid absorption and metabolism in the intestine.
Taken together, the diet-responsive regulation of vitamin A production provides an elegant mechanism to cope with seasonal fluctuations in food supplies and unbalanced diets.
Researchers showed that an enzyme in the intestines, where carotene is converted to vitamin A, is part of this elegant feedback loop. The transcription of this protein, BCO1, the enzyme that helps convert beta-carotene, is downregulated in the intestines via the factor ISX (intestine-specific transcription) as more vitamin A is formed. The ISX protein is formed under the influence of the transcriptional activator retinoic acid (RA). In this way, too much retinol is prevented from being formed from beta-carotene. 19)
On the other hand, the ingested retinol (vitamin A) is converted to retinyl esters and stored in the liver, which reduces the chance of acute poisoning. With the enzyme lecithin retinol acyltransferase (LRAT), the excess retinol (ROL) is converted to retinyl esters (RE). The transcriptional activator retinoic acid (RA) determines the transcription of the protein LRAT.
Similarly, RA also controls the uptake and storage of vitamin A by inducing the expression of LRAT which sequesters retinol as REs. 20)
LRAT transforms ROL into its inert ester form and prevents toxic and teratogenic effects of the nutrient, as we previously showed in a zebrafish model. 21)
Covert Poisoning
Why then go the unsafe route with synthetic retinyl esters as additives..? In this way the limiting control mechanism in our body is circumvented. It cannot then maintain equilibrium, no homeostasis,.
Infobox: the effects of vitamin A versus betacarotene
Vitamin A can be dangerous during pregnancy; high doses or accumulated levels can be teratogenic. It can cause birth defects.
The teratogenicity of vitamin A may depend on an excessive dose during critical periods of fetal development that transiently overwhelms maternal transport and homeostatic mechanisms. Conversely, vitamin A has a strong tendency to bioaccumulate, and hence there is a possibility that excessive intake of vitamin A during the weeks or months preceding conception may contribute to teratogenic risk. 22)
This teratogenic effect goes for vitamin A, which is a retinoid. It does not apply to carotene:
Experiments in animals have shown that retinoids (but not carotenoids) can be teratogenic.
These data appear to indicate a teratogenic effect of vitamin A at levels not far above those currently recommended. 23)
In contrast, the conversion from beta-carotene seems to be regulated and limited by the body:
Experimental studies in animals have shown that β-carotene is not mutagenic or teratogenic. Doses up to 180 mg of β-carotene per day have been used for many years to treat patients with erythropoietic protoporphyria, with no evidence of vitamin A toxicity and without the development of abnormally elevated blood vitamin A concentrations. This is due to the observation that conversion of β-carotene and other provitamin A carotenoids seems to be regulated by the vitamin A status of individuals. Therefore, high intakes of carotenoids do not lead to abnormally high vitamin A concentrations or symptoms of hypervitaminosis A. 24)
When “preformed vitamin A” is taken directly, the feedback mechanism is bypassed, incurring a risk of poisoning:
It is important to note, that a negative feedback mechanism does not appear to operate in the case of the intestinal uptake of dietary preformed vitamin A (retinol, retinyl esters) which incidentally are associated with a much higher risk of teratogenicity and toxicity compared to provitamin A carotenoids. 25)
In 2015, the European Food Safety Authority (EFSA) established nutritional standards for vitamin A and adjustments were made to European legislation for vitamin A that would protect consumers when eating organ meat. From now on, vitamin A could not simply be supplied to animals for consumption through drinking water. But the same consideration immediately offered an opening for the sector to do so anyway, via a “compound feed”.
Vitamin A should not be administered directly via water for drinking because an additional route of administration would increase the risk for consumers. Therefore, the authorisation of retinyl acetate, retinyl palmitate and retinyl propionate as nutritional additives belonging to the functional group ‘vitamins, pro-vitamins and chemically well-defined substances having similar effect’ should be denied as regards their use in water. This prohibition does not apply to those additives within a compound feed subsequently administered via water. 26)
The body can still store the excess retinoids in the liver and prevent acute poisoning. But how long will that last? After all, vitamin A cannot continue to accumulate in the liver, which does not have an unlimited absorption capacity. This quote from the research mentioned below summarizes it aptly. It goes well until it goes wrong.
The cleavage of provitamin A carotenoids to retinal is a highly regulated step, and vitamin A toxicity from provitamin A sources is largely impossible. In contrast, absorption and hepatic storage of preformed vitamin A occur very efficiently until a pathologic condition develops. 27)
Infobox: deadly sensitive to vitamin A
This 1987 article discusses the case of a toddler whose intake of this unique vitamin was found to be too high. He died of vitamin A poisoning at the age of three.
A 2-year-old boy had signs and symptoms of chronic hypervitaminosis A. A
course of increasing severity led to eventual death. 28)
Infobox: the case Carrot Juice Junkie
In 1983, retired researcher Dr. Charles W. Marshall (PhD) published the book “Vitamins and Minerals: Help or Harm?” in which he describes the case of a nutritionist who fatally poisoned himself. 29)
His doctors warned him about the dangers of excessive vitamin A and cut off his access to vitamin A from the dispensary. But Gerald just could not believe that anything as “wholesome” as a vitamin could really have made him sick. After brooding a few days about the injustice of being disallowed his favorite vitamin, he persuaded his wife to prepare fresh carrot juice daily using a blender.
The excess amounts of carotene from the carrot juice turned his skin yellow, but the nutritionist died of liver failure as a result of the earlier vitamin A poisoning. He only lived to be 48 years old.

Two Routes
There are two ways in which we can obtain this important, endogenous substance vitamin A:
produce it ourselves from plant foods, the “pro-vitamins” (carotenes),
as a “preformed vitamin” from animal sources, or as synthetic esters.
A sufficient but limited supply is created in the liver via the first route. This limitation makes this route very safe. A feedback mechanism ensures that no more carotene is converted than is necessary to serve as a buffer stock. Through this negative feedback, the retinol level in the blood determines what happens to the carotene in the intestines. 30)
The second route also allows some retinoids to be taken directly without the risk of acute poisoning. Any surplus is added to the stock in the liver, which then serves as safe storage. However, the rapid storage of retinoids and relatively slow elimination can lead to liver toxicity. The toxicity can take years, possibly decades, to build up. Also, ingestion by this route will lead to elevated blood levels. 31)
Infobox: the effect of long-term vitamin A accumulation
In addition, retinol has a long biologic half-life and bioaccumulates. The combination of relatively rapid absorption with a low clearance can produce acute toxicity within hours after a sufficiently high dose and chronic toxicity after prolonged intake of substantially smaller doses. 32)
Chronic toxicities occur after several weeks, months, or years of consumption of lesser amounts that are not acutely toxic. 33)
Therefore, taking all the studies together, it is clear that vitamin A supplementation does increase serum retinol levels. Not only was there a doseresponse relationship within our own study, but across all the studies those which gave the largest doses of vitamin A observed the largest increases in serum retinol. 34)
Transcriptional Activation
It was only in the late 1980s that it began to dawn that vitamin A plays a direct role in translating our genetically coded material into proteins. This is an extremely important process, for example for reproduction; therefore during pregnancy and embryonic development.
Retinoic Acid, the final metabolite in vitamin A metabolism (see picture above), activates a nuclear receptor in our cells: the RAR. This activation initiates transcription, the translation of genetic codes into the production of proteins, or gene expression.
Without vitamin A, there is also no retinoic acid and no transcriptional activation. Without an activating metabolite, there will be no building blocks for the baby. In the case of a deficiency (avitaminosis), reproduction logically goes wrong. With birth defects as a result. But with too much, so in case of hypervitaminosis A, things also go wrong! Vitamin A then suddenly becomes teratogenic! Thomas Moore described it in his voluminous book from 1957:
Hypervitaminosis A resembles avitaminosis A in interfering with reproduction, and the ranges of congenital abnormalities which can be incurred seem to be much the same. 35)36)(p549)
“Vitamin A is essential.” We need to have it in our bodies. But just a little too much and it's wrong again!? How?
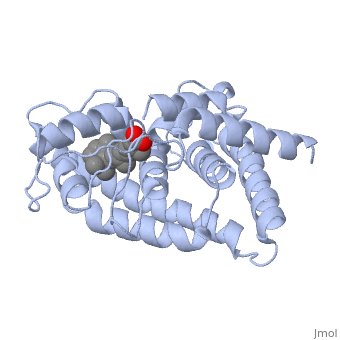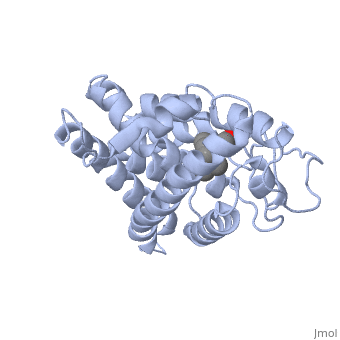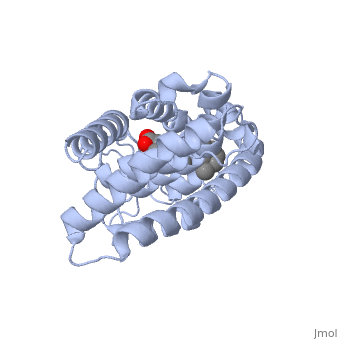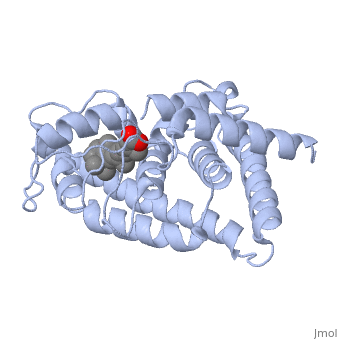
Infobox: activating versus blocking the vitamin A receptor
The molecule all-trans retinoic acid (ATRA) fits exactly into the nuclear receptor RAR, which it activates. The rotating image shows how and where exactly that retinoic acid molecule is located in the receptor. This position can be determined very accurately with molecular modeling in software. Note the two red oxygen atom balls in the animation! The retinoic acid (RA) is the activating ligand. Without this retinoic acid molecule there is no activation. And without vitamin A, there are no retinoic acid molecules. 37)
There is a major difference between the beta-carotene and vitamin A (retinol) molecules. The chemical structures of both substances were determined by Paul Karrer in the early 1930s. 38)39) Thomas Moore was therefore able to neatly draw them in his 1941 article.

It is immediately apparent that vitamin A is about half a beta-carotene. In 1941, Thomas Moore was not able to include the other retinoids from vitamin A metabolism as these were yet to be discovered. These other retinoids are all very similar to retinol and, like retinol itself, are also about half the size of beta-carotene. The molecules retinol and retinal both have only one oxygen atom, while retinoic acid has two.
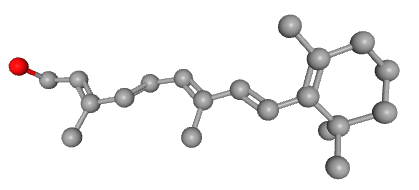
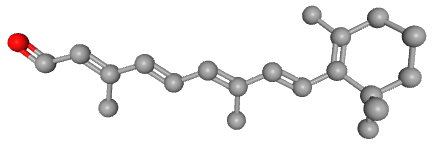
This small difference and at the same time the very similar shape suggests that they could disrupt gene expression in the receptor. Because both molecules are ligands for the RAR, they fit into that receptor. However, retinal has a 500x lower affinity (it is 500x less attracted) than retinoic acid. But retinol only has 35x less affinity. We can therefore not rule out that retinol molecules bind to that receptor, which could explain the teratogenicity. 40) After all, if we raise the blood level of retinol through a high intake or accumulation of vitamin A and the retinol therefore binds to the RARs, then the retinoic acid cannot sufficiently activate the receptor and gene expression is disrupted.
A molecule twice the size, such as beta-carotene, will logically not fit into the receptor. It will therefore never be able to disrupt gene expression in this way, not even at high concentrations. Retinoids, on the other hand, can do this and are much more unsafe. It is even “difficult to find efficacious pharmacological retinoids that are not teratogenic”. 41)
From the same study is also this statement:
Unlike the retinoids, which are stored in the liver, carotenoids are stored in adipose tissue, skin, liver, adrenal gland, and testes. Extremely large doses of β-carotene have not been teratogenic to chicks, rats, or rabbits. Case reports indicate that β-carotene has not been teratogenic in humans.
It makes quite a difference how we get our vitamin A. We can make it ourselves very safely from carotene or, with a certain risk, consume it directly as retinol or as a retinyl ester. In the first case, the conversion to vitamin A is limited to a safe level, while in the second case it can increase more and more.
There are good reasons to meet our need for this very important, yet endogenous substance “vitamin A” by simply eating tomatoes, carrots, kale and spinach!!! 💪💪💪
Read more about vitamin D ☛












